











REDESIGNED BAG VALVE MASK
SUMMARY:
I am currently part of a team of 8 undergraduate bioengineering students and nursing students as part of a two semester senior design capstone project in medical device design, ideation, and development. Through this project our team participated in extensive ethnographic efforts to uncover unmet clinical needs to develop solutions for. The arrived at unmet clinical need was developing a better fitting and sealing bag valve mask (BVM) face mask. The best concept to develop was to redesign the current BVM face mask with a foam cushion instead of the standard air filled bags. Throughout the development of the BVM, our senior design capstone gave us exposure to the FDA medical device design process and to create associated documents and perform associated activities in tandem with the prototyping process. Such documents and activities include developing ethnography plans, protocols, and test reports; conducting hazard and risk analysis on our solution; and developing PDS documents to list technical specifications. All documents were organized and contained in a comprehensive DHF. Formal Verification and Validation (V&V) testing was conducted our "production equivalent" prototypes.
RESEARCH GROUP MEMBERS:
-
Zachary Roy
-
Pranay Jain
-
Arijit Dutta
-
Minna Kuriakose
-
Noah Ziemba
-
Jacob Atkinson
-
Joseph Madill
-
Isabel Morris
TIME INVOLVED:
-
August 2021 - April 2022
CLASS INFORMATION:
-
Class: BIOENG 1160 & 1161
-
Class Professor: Dr. Mark Gartner
SKILLS LEARNED: Ethnography experience (Observational Analysis, Task Analysis, Interviews), sketching and low-resolution prototyping activities, laser cutting and medium-resolution prototyping experience, FDA Medical Device regulation and associated documentation experience.
DEVICE DEVELOPMENT
Ethnographic Efforts and Problem Discovery
For the first month of the course, focus was placed on unmet clinical need discovery through interviews. During the initial week, I and the rest of my team individually interviewed 5 different clinicians each in a wide variety of medical disciplines and fields such as P&O, Emergency Medicine, Nursing, Surgery, General Medicine, etc. Collection and discussion of ideas condensed the project into 4 potential areas of focus: medical professional mental health, medical education and simulation, emergency medicine services, and general medicine.
With these 4 concentrated areas in mind, I helped construct ethnography plans and protocols for each area of interest. These documents would be used to conduct specific ethnographic efforts in each setting like task analysis, observational analysis, and interviewing.
Through these ethnographic efforts, I helped identify several specific issues in the emergency medicine and general medicine applications. These included poor and uncomfortable design of bedpans that led to spilling, poor fitting and sealing of bag valve masks, and insufficient fitting of fingertip pulse oximeters.
Focused ethnographic efforts to two local EMS stations, the University of Pittsburgh Emergency Medicine Department, the WISER medical simulation lab, and several clinician interviews revealed that poor fitting and sealing of bag valve masks in emergency medicine applications was an issue we heard most frequently about, specifically with less-experienced EMTs/Paramedics in instances where only one person was available to bag a patient.
We also heard how it was a fairly frustrating issue because oxygen could not be delivered efficiently to the patient and the current fitting caused substantial hand fatigue to the user as bagging a patient should go on as long as necessary until the patient is is able to breathe on their own again or when they are intubated.
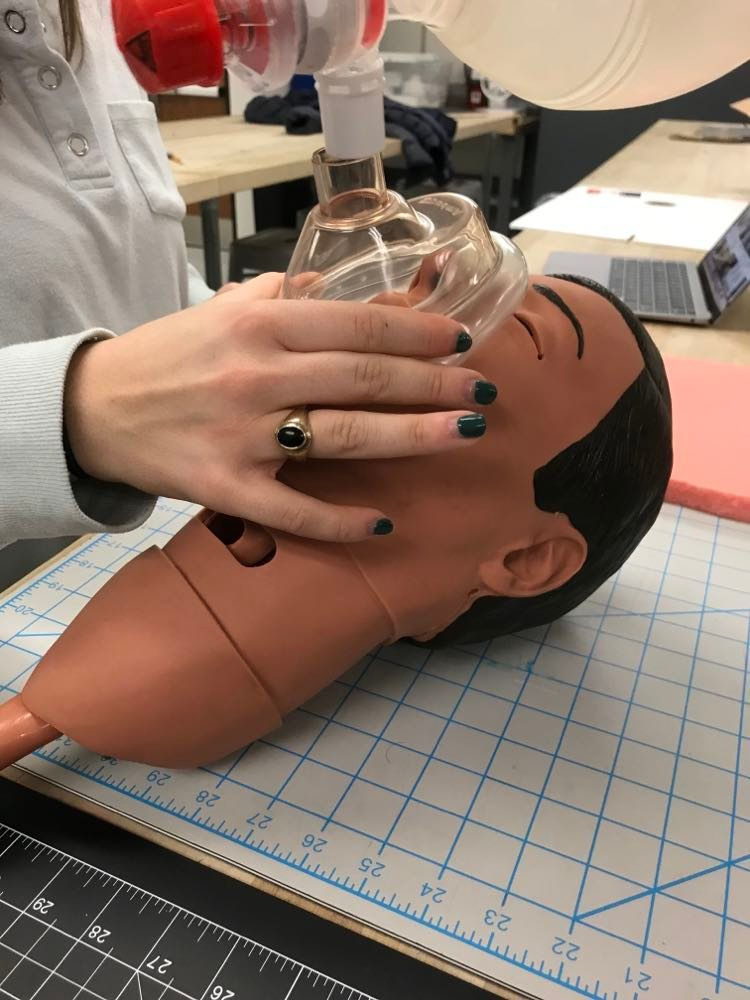
Isabel Morris, our nursing student, demonstrating a 1 handed sealing of a BVM face mask on a CPR manikin head. The other hand will be used to squeeze the ventilation bag
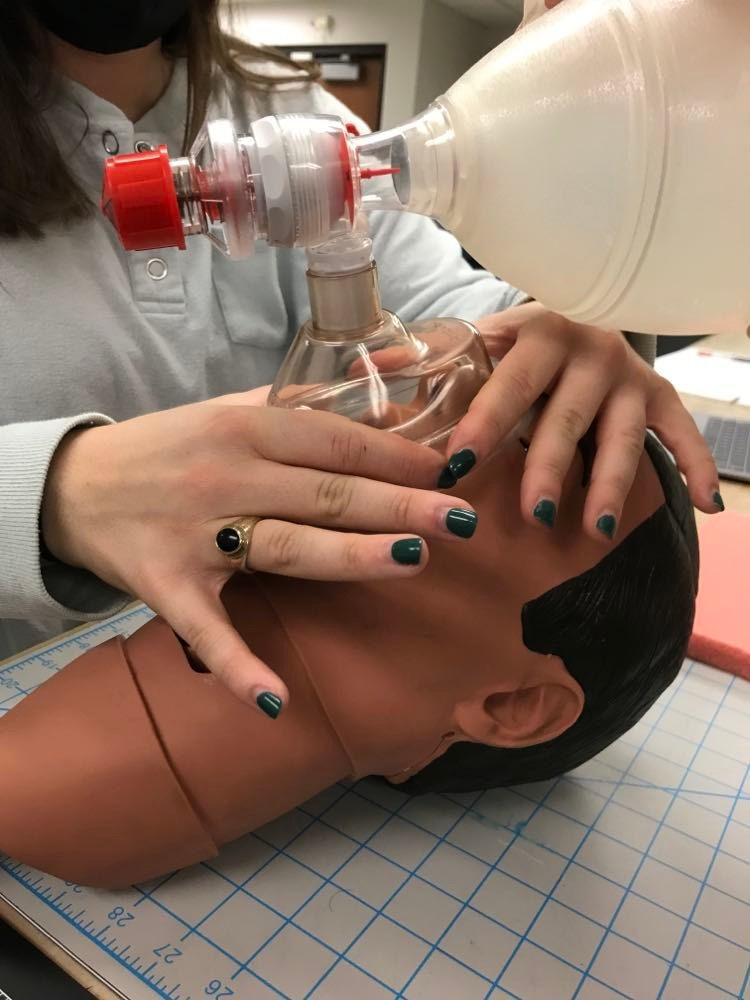
Isabel Morris, our nursing student, demonstrating a 2 handed sealing of a BVM face mask on a CPR manikin head. Another person would be squeezing the ventilation bag.

Isabel Morris, our nursing student, demonstrating a 1 handed sealing of a BVM face mask on a CPR manikin head. The other hand will be used to squeeze the ventilation bag
Group Brainstorming, Sketching, and Killer Experiments on MVPs
With the problem clearly defined, our group set out on brainstorming various ideas and concepts independently. I was in charge of compiling all ideas and loose sketches from our 7 teammates. I then quickly developed more detailed drawings to use as minimum-viable-prototypes (MVPs) for our continued meetings with two local EMS stations (Foxwall EMS, Shaler Hampton EMS), the Pitt Emergency Medicine Department, and various interviews with emergency medicine instructors and doctors.
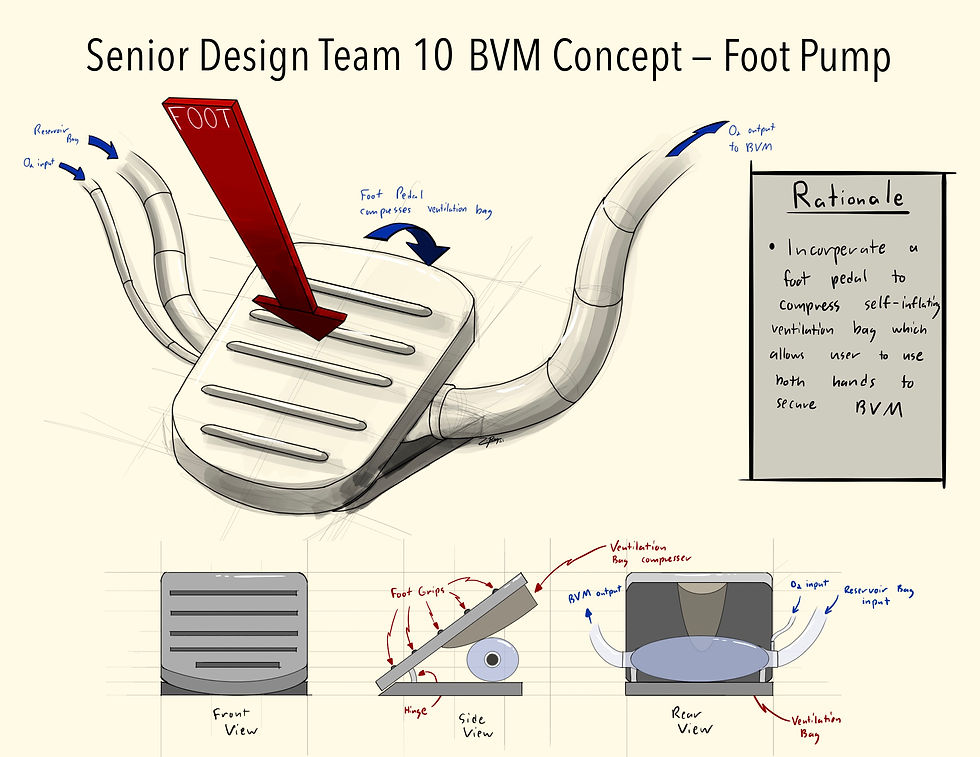
This is a concept sketch of a ventilation bag foot pedal. The incorporation of the foot pedal would compress the self-inflating ventilation bag. This would allow the user to use both hands hold and create a better seal with the BVM face mask.

This is a concept sketch of a face mask pressure distributor. The incorporation of the pressure distributor would allow the user to distribute the pressure of their hand evenly across the BVM face mask via a handle. This would be idea for EM personnel with smaller hands.

This is a concept sketch of attachable ear hooks to an existing BVM face masks. The incorporation of the ear hooks and straps would secure the BVM on the face, creating a tighter seal on the face in addition to one hand sealing of the BVM face masking. The ear hooks would be adjustable to accommodate for varying ear sizes and shapes.

This is a concept sketch of a ventilation bag foot pedal. The incorporation of the foot pedal would compress the self-inflating ventilation bag. This would allow the user to use both hands hold and create a better seal with the BVM face mask.
FEEDBACK ON MVP SKETCHES
I with members of my team presented these sketches to Foxwall EMS and the Pitt EM Department for feedback on the validity of our ideas and to conduct some "killer experiments' to quickly kill concepts that are less likely to succeed.
The EM personnel we spoke with communicated that BVM masks should be easily removable in the scenario where a patient would vomit or something else would compromise the patients ability to breathe within the BVM face mask. Because of this, they strongly advised against the ear hook concept, which allowed us to kill the ear hook concept.
The EM personnel also strongly preferred a solution that did not require additional steps to the current bagging process as this would require additional training. The time to implement these steps would cause a hazard by leaving the patient unventilated for a longer period of time. Because of this, the foot pump concept was killed as well. Preference was given to the pressure distributor and the memory foam concepts.

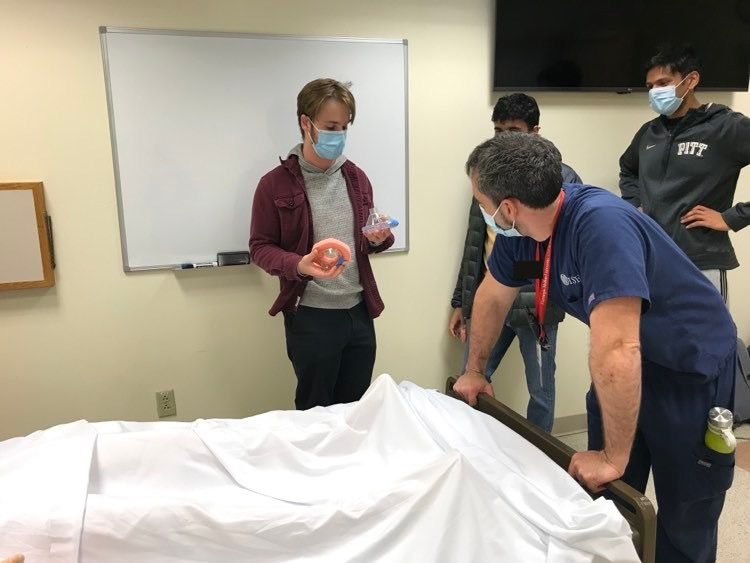
This is a sketch of our team showing our MVP drawings to EM medicine professional. The man holding the paper in the photo is an EM instructor for the Pitt EM department. I am standing to the left of him pointing out details of our concepts. Fellow teammates, Jake and Pranay, are also shown.

This is a sketch of our team showing our MVP drawings to EM medicine professional. The man holding the paper in the photo is an EM instructor for the Pitt EM department. I am standing to the left of him pointing out details of our concepts. Fellow teammates, Jake and Pranay, are also shown.
Specifically for the pressure distributor, the EMT would position their hands to "bring the face up into the mask" rather than "push the mask on the face" so our design would have to reflect this. Creating the mask with a foam cushion rather than an external attachment would also be preferred given the attachment point could create an improper seal and attaching the foam would add an additional step.
Due to the complexity of the pressure distributor's new design and the time frame of our project, the pressure distributor concept was killed by the team. Focus was then placed on creating low-resolution prototypes of the memory foam concept.
Low-Resolution Prototype Development and Feedback

This is low resolution prototype is a standard BVM face mask with a ring of medium-density memory foam inserted into the cushion pocket.

This is low resolution prototype is the top portion of a standard BVM face mask attached to a ring of raw medium-density memory foam as the cushion. The cushion is approximately 0.5" wider on all points.

This is low resolution prototype is a standard BVM face mask with gel beads inserted into the cushion pocket.

This is low resolution prototype is a standard BVM face mask with a ring of medium-density memory foam inserted into the cushion pocket.
LOW-RESOLUTION PROTOTYPES
I and members of my team began constructing low-resolution prototypes of the memory foam mask concept. Three different variations were constructed with different types of cushioning. On all three prototypes, the new cushion would provide less resistance than the standard air-filled cushions and provide a better contoured fit, thus improving seal and handing of the mask.
LOW-RESOLUTION PROTOTYPE FEEDBACK
I along with some members of my team presented our three low-resolution prototypes to medical professionals at the Pitt WISER Medical Simulation Lab once again for feedback and informal testing on CPR manikins. This feedback showed that the gel bead and wide foam masks required less pressure to create a sufficient seal on the face, potentially reducing hand fatigue.
Risk documentation was developed around these prototypes such as an initial hazard analysis (IHA), risk summary, fault tree analysis (FTA), and failure modes and effect analysis (FMEA). A hazard identified in the device was tearing of the plastic coating surrounding the filling material. In the case of the gel mask, this could cause release of gel into a patient’s mouth. This hazard is not present when using foam, so our team decided to pursue a foam-based mask to reduce risk in our design.

Images of Low-Resolution Prototype Feedback and Informal Testing
Images of Low-Resolution Prototype Feedback and Informal Testing

Image of Low-Resolution Prototype Feedback and Informal Testing

Images of Low-Resolution Prototype Feedback and Informal Testing
Additional feedback with an emergency medicine doctor at UPMC and from paramedics at Shaler Hampton EMS suggested a longer cushion that is softer or contained multiple types of foam. This feedback and design suggestions showed that we were on a good path with our general solution but had some design revisions to consider. This would lead us to our medium-resolution prototyping of the foam BVM mask.
Medium-Resolution Prototype Development and Feedback
MEDIUM-RESOLUTION PROTOTYPES
My responsibility during the last week of the semester prior to finals was to construct medium-resolution prototypes. Our group had acquired two densities of foam: a pink medium-density memory foam and a green low-density urethane foam.
To get the foam mask shape, I measured the standard cushion with dial calipers and used the measurements to create CAD sketch in Fusion 360. With the CAD file, I converted it to a DXF file to then laser cut the foam rings out on a laser cutter in one of our innovation workshops (Pitt Makerspaces). I also laser cut a cardboard top for stability and even dispersion of force when the hand is placed on the mask.
The layers were adhered together via spray adhesive. The top parts of the BVM face masks was cut from standard BVM masks ad adhered to the cardboard via Silicon glue.
All four concepts have the same basic concept but different variations, similar to the Low-Resolution Prototyping Round.
MEDIUM-RESOLUTION PROTOTYPE FEEDBACK
Informal testing and feedback on the medium-resolution prototype was conducted during January 2022 and February 2022 at some local EMS stations, WISER, and the Pitt EM Department. The results from these visits showed that the mask with the large green low-density foam was most preferred for comfort and functionality.

This medium-resolution prototype contains two layers of foam with differing densities. The pink foam is a medium density memory foam. The green foam is a low-density urethane foam. It is of standard mask dimensions.

This medium-resolution prototype contains two layers of foam with differing densities. The pink foam is a medium density memory foam. The green foam is a low-density urethane foam. It's cushion width is 0.5" greater on all sides.

This medium-resolution prototype contains one deep density of green low-density urethane foam. It's cushion width is 0.5" greater on all sides.

This medium-resolution prototype contains two layers of foam with differing densities. The pink foam is a medium density memory foam. The green foam is a low-density urethane foam. It is of standard mask dimensions.
"Production Equivalent" Test Prototype Development
"PRODUCTION EQUIVALENT" TEST PROTOTYPES
After the previous round of informal feedback with the four variations of the foam mask, I began constructing our team's final engineering test prototype. This prototype is comprised of I began by modifying the Fusion360 file to fit new size dimensions. Using these dimensions, I laser cut the foam cushions using a Rabbit Laser Cutter in our engineering building's Makerspace. I also laser cut the hand-supporting plates out of 1/8" polystyrene.
The foam cushion, supporting plate, and a hard plastic upper transplanted from a standard Ambu Medium Disposable BVM were adhered together with an adhesive spray. To make sure the foam would be resistant to fluid and prevent airflow into it, a protective coating was applied to the foam. Firstly, I tried a Plastic-Dip rubber coating. After applying, it was revealed that it made the foam much too rigid and prevented an adequate seal from forming. Because of this, I switched to using Dragon Skin 10, a flexible silicone coating. This coating proved to make the foam much less rigid but was slightly less durable. However, informal verification testing the mask seemed to work well. With the final design completed, our team then began formal verification and validation testing.
The images in the slideshow on the right document some moments during our prototyping process.

Here I am laser cutting the polystyrene hand support plates using a Rabbit Laser Cutter in our engineering building's Makerspace.

Here are me (left) and fellow teammate, Minna, applying a Plasti-Dip coating to the foam cushions.

After the Plasti-Dip coated masks did not work, our team shifted to Silicone coating which was much more flexible. Here I (left), am testing the new mask prototype with fellow teammate, Jake.

Here I am laser cutting the polystyrene hand support plates using a Rabbit Laser Cutter in our engineering building's Makerspace.


FINAL "PRODUCTION EQUIVALENT" PROTOTYPE DESIGN
Here is a diagram of our team's final prototype showing the components and design of final test prototype. The final prototype is composed of a hard plastic BVM upper, a rigid polystyrene hand-supporting plate, a low-density urethane foam cushion coated in flexible silicone.
Verification and Validation (V&V) Testing and Results
VERIFICATION TESTS
Our team conducted various verification tests focused on functionality and meeting our design specifications. The four verification tests our team conducted were:
-
VER-01: Testing functionality of the prototype on a CPR Manikin (SimMan3G) that showed a readout of tidal volume in the artificial lungs.
-
VER-02: Measuring the density of the prototypes
-
VER-03: Assessing the fluid resistance of our prototypes
-
VER-04: Comparing weight and spacial dimensions of our prototype and a standard mask
My primarily involvement was the collecting of data for VER-01 and the entirety of VER-04. Data for VER-01 was collected at Pitt WISER Medical Simulation Lab using a high tech CPR Manikin that showed the tidal volume readout of the lungs, which shows how well our mask was able to ventilate the patient. Simulation instructors and other WISER staff were recruited to test our device on the manikin which provided us the quantitative data.

SimMan3G used to obtain tidal volume data to test the functionality of our prototypes


SimMan3G used to obtain tidal volume data to test the functionality of our prototypes
For VER-04, I was in charge of writing the Verification Test Plan, Test Protocol, and Test Report as well as conducting the test. One of the key design criteria was to create a solution that did not add any new steps to any protocol EM personnel would perform. Creating a mask that was outside standard dimensions could make the device a hinderance and impact its overall functionality. In this test, I measured the weight, overall height, overall length, and overall width of the prototype masks and the standard mask. After conducting this test, it showed that the prototype masks were heavier and the uncompressed dimensions were outside of the standard range.
VALIDATION TESTS
Our team conducted validation tests by visiting several EMS Stations in the Greater Pittsburgh Area and obtaining formal feedback from EM personnel. During our tests, EMTs and Paramedics would test our our mask on a CPR manikin and provide quantitative and qualitative feedback via a questionnaire. By doing this, we were able to get data from ~20 EMTs at 5 different EMS locations.
V&V TEST RESULTS
For results, the device failed functionality and fluid resistance testing due to leaks in the silicone coating. Use of new materials and geometry meant that the device also failed the established dimensional and material specifications. However, all three validation tests did pass. Providers cited an improvement in mask seal and reduction in fatigue while using the mask. In the future, the clinical effectiveness of the mask could be enhanced by improving the coating.
Application to FDA Regulatory Documents
In addition to developing a medical device to solve an identified clinical unmet need, the purpose of this course is to familiarize us with the FDA medical device development process in accordance to 21 CFR 820.30 (Design Controls). Our risk analysis and mitigation strategies are also guided by ISO 14971. Our design process was then comprised in a comprehensive design history file (DHF). Education on product codes and risk. In accordance with FDA Design Controls, our group constructed and continually revised the following "living" documents:
-
Ethnography plans for each field of interest
-
Ethnography protocols for each field of interest
-
Ethnography test reports for each conducted activity
-
Initial Hazard Analysis (IHA)
-
Fault Tree Analysis (FTA)
-
Failure Mode Effect Analysis (FMEA)
-
Risk Summary
-
Product Design Specification (PDS)
-
Traceability Matrix
-
Use Case, Intended Use, and User Needs documents
-
Verification Test Plans
-
Verification Test Protocols
-
Validation Test Plans
-
Validation Test Protocols
-
Header and Methodology for Document Control and Revision